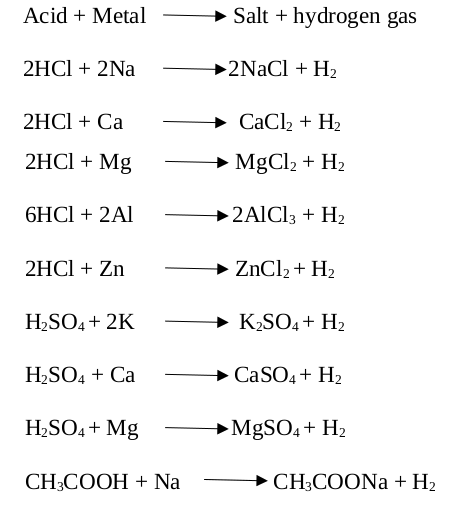
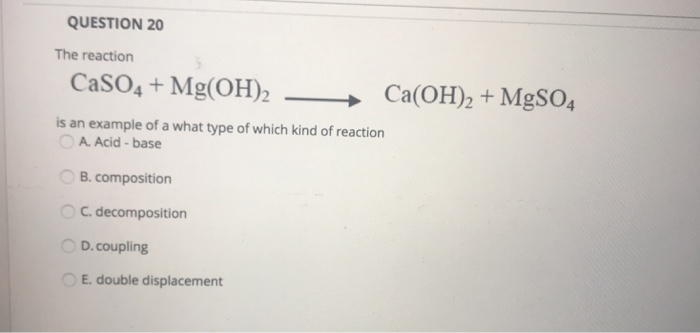
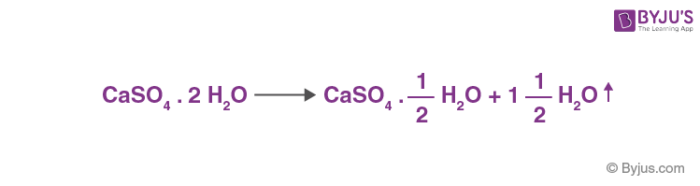
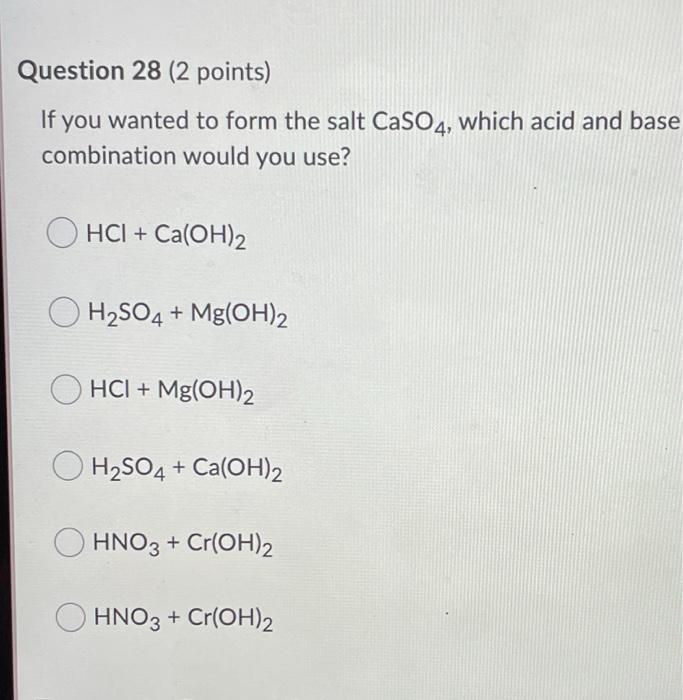
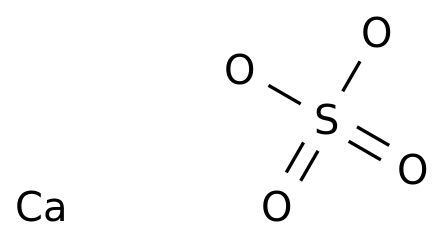
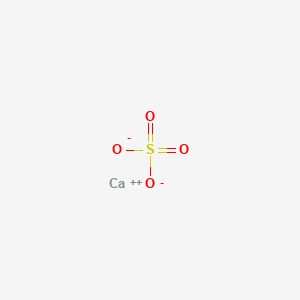
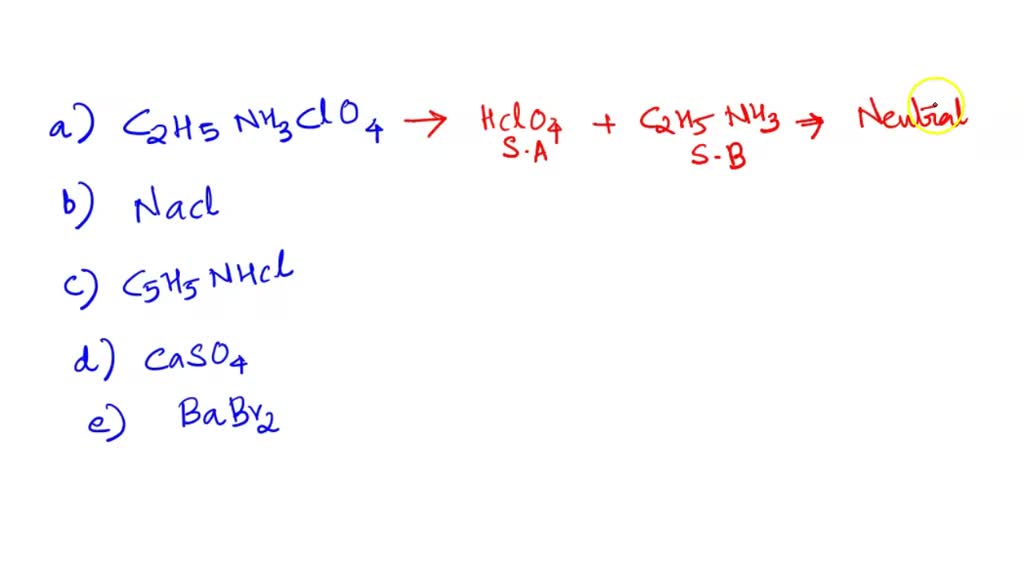What is the nature of the salt CaSO4 formed by the reaction between calcium hydroxide and sulphuric acid?
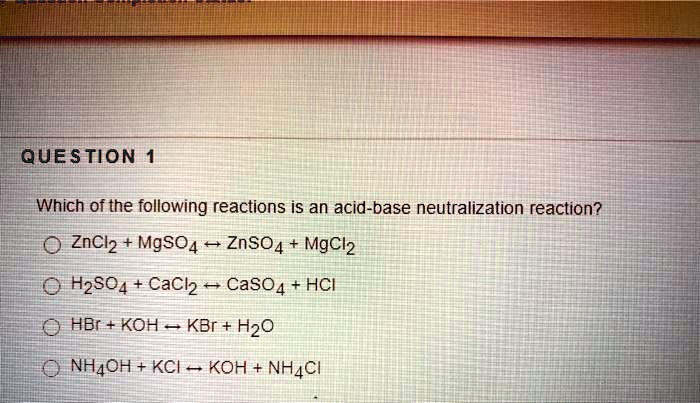
SOLVED: QUEStioN Which of the following reactions is an acid-base neutralization reaction? 0 ZnClz + MgsO4 ZnSo4 MgCl2 0 H2S04 + CaClz CaSo4 + HCI HBr KOH = KBr H20 NHAOH + KCI = KOH + NHACI

4. Circle all the pairs of compounds below that represent a conjugate acid base pair. a. H2S/HS- b. - Brainly.com

Preparation and Application in HDPE of Nano-CaSO4 from Phosphogypsum | ACS Sustainable Chemistry & Engineering