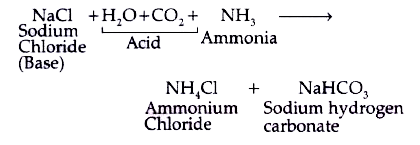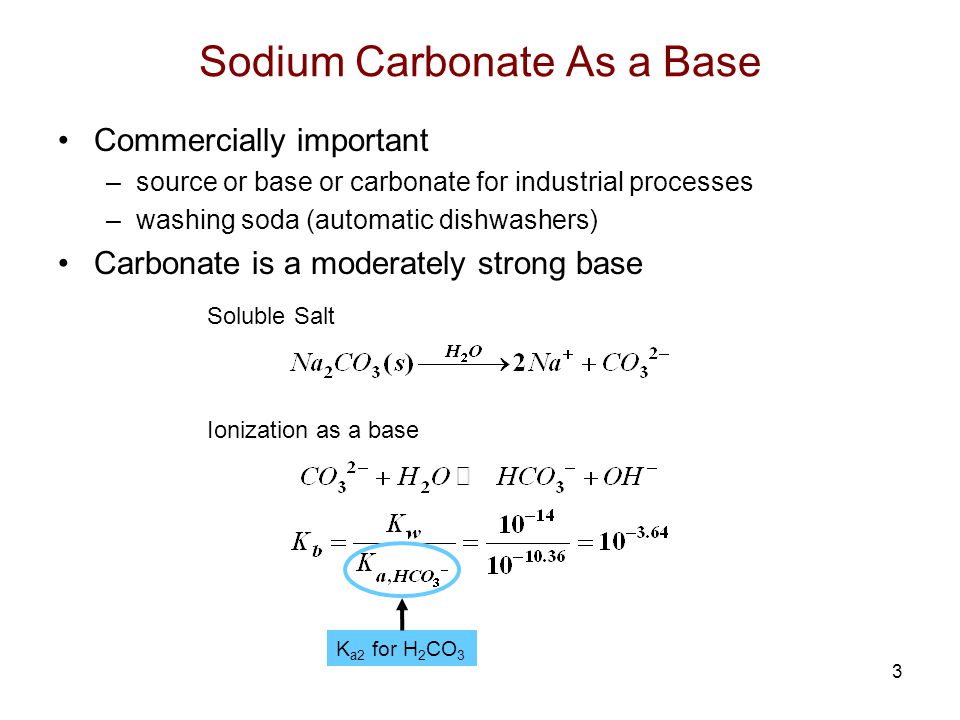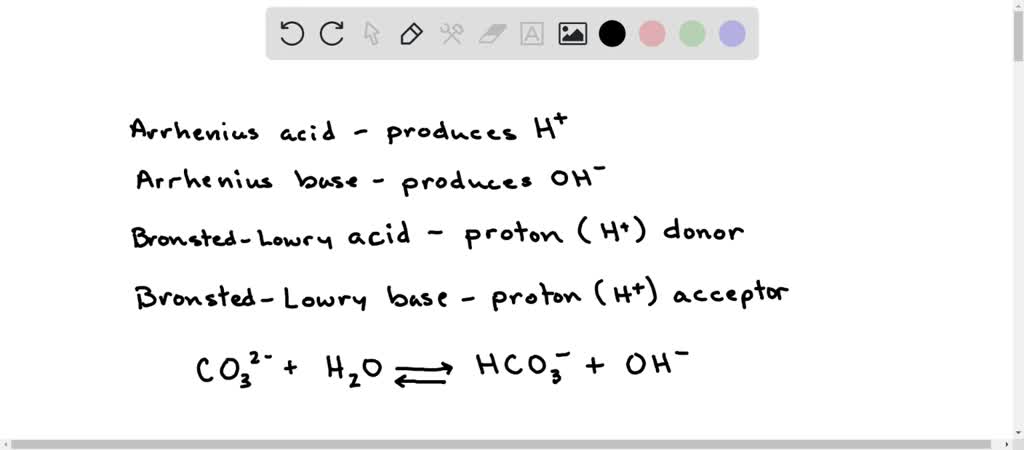
SOLVED: In the reaction CO3 + H2O â†' HCO3- + OH-, the carbonate ion is acting as a(n) Arrhenius base and a Bronsted-Lowry base.

Write a mechanism (using curved-arrow notation) for the deprotonation of tannins in base. Use Ar-OH as a generic form of a tannin and use sodium carbonate (Na2CO3) as the base. Balance the

Reactions. DON'T COPY pink WRITING. Acid + base This is a common reaction and needs to be remembered for exams. ACID + BASE -> SALT + WATER E.g. hydrochloric. - ppt download





![MCQ] - Which correctly represents Parent acid and base of Calcium MCQ] - Which correctly represents Parent acid and base of Calcium](https://d1avenlh0i1xmr.cloudfront.net/0014c0c3-c848-4073-a814-6e71c0e2bf5e/reaction-to-form-calcium-carbonate---teachoo-01.jpg)
.PNG)