Catalytic Hydroboration of Aldehydes, Ketones, and Alkenes Using Potassium Carbonate: A Small Key to Big Transformation | ACS Omega
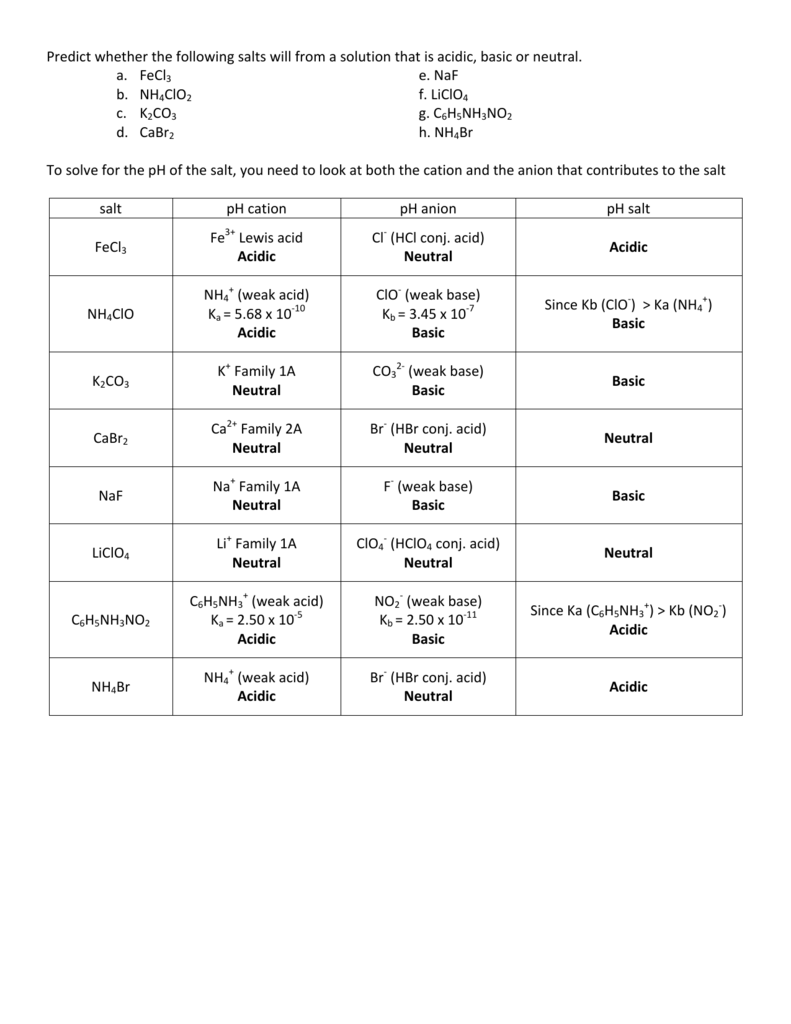
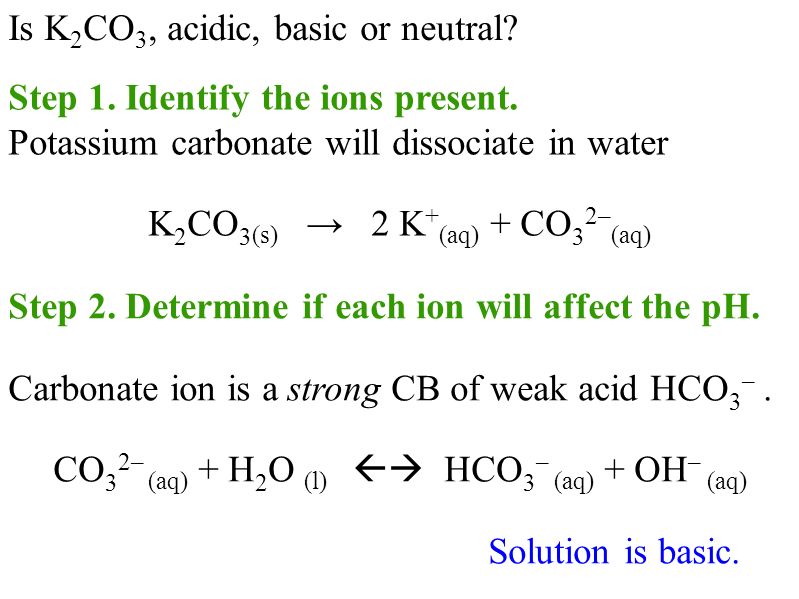
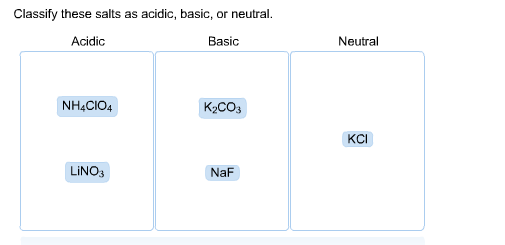
SOLVED: Select the salt from the list below which will produce a basic aqueous solution. Group of answer choices NaNO3 K2CO3 NH4Cl K2SO4
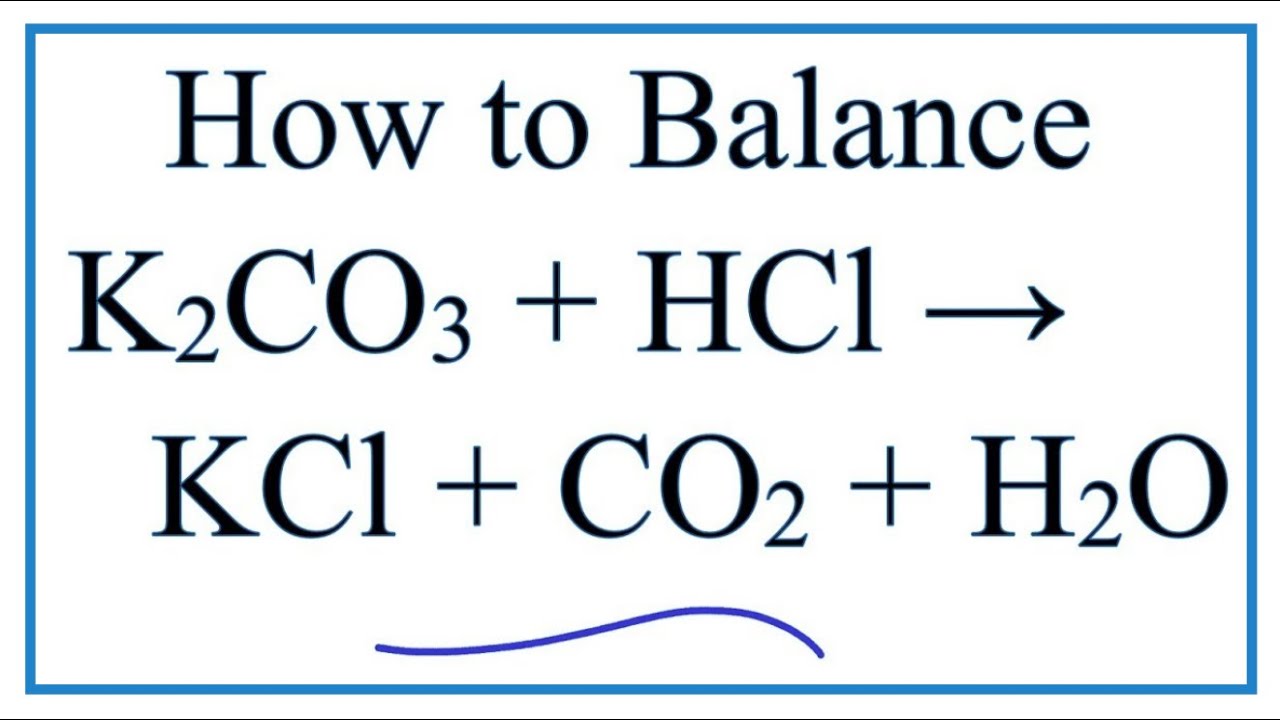
In the reaction between potassium carbonate and hydrochloric acid, 25 grams of salt were produced. If excess acid was available, how many grams of the carbonate were used? - Quora