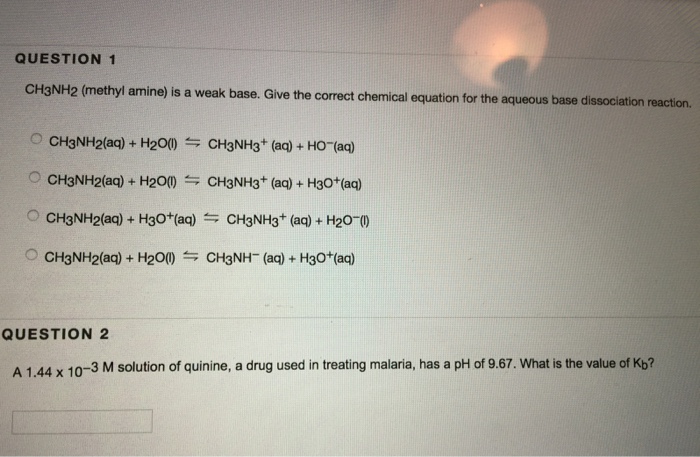
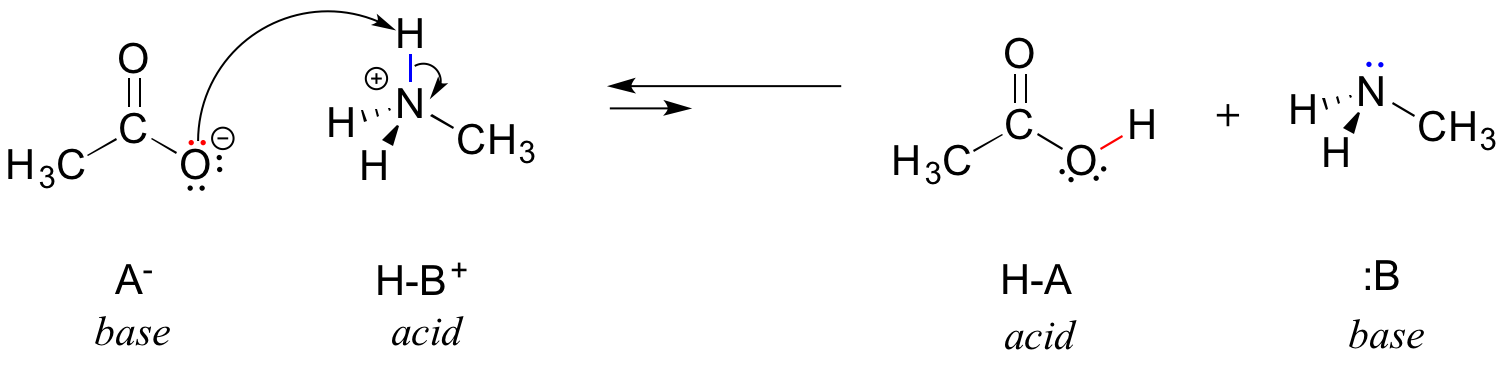
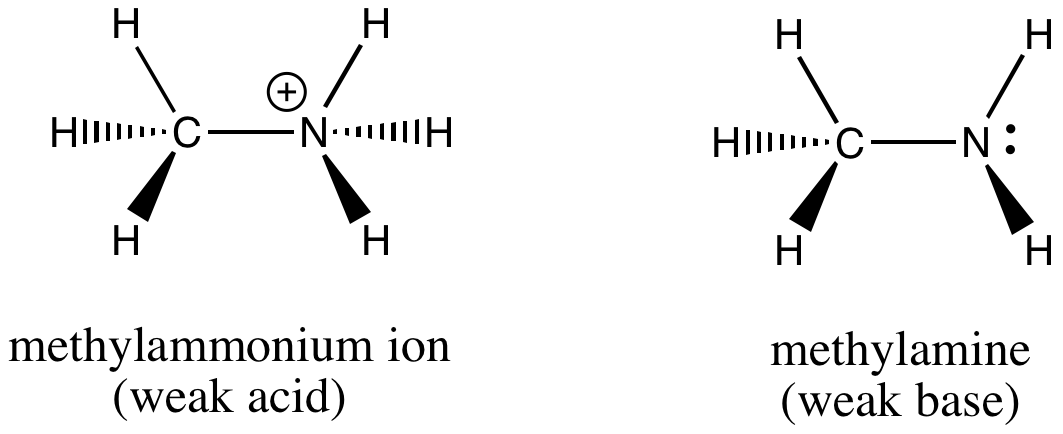
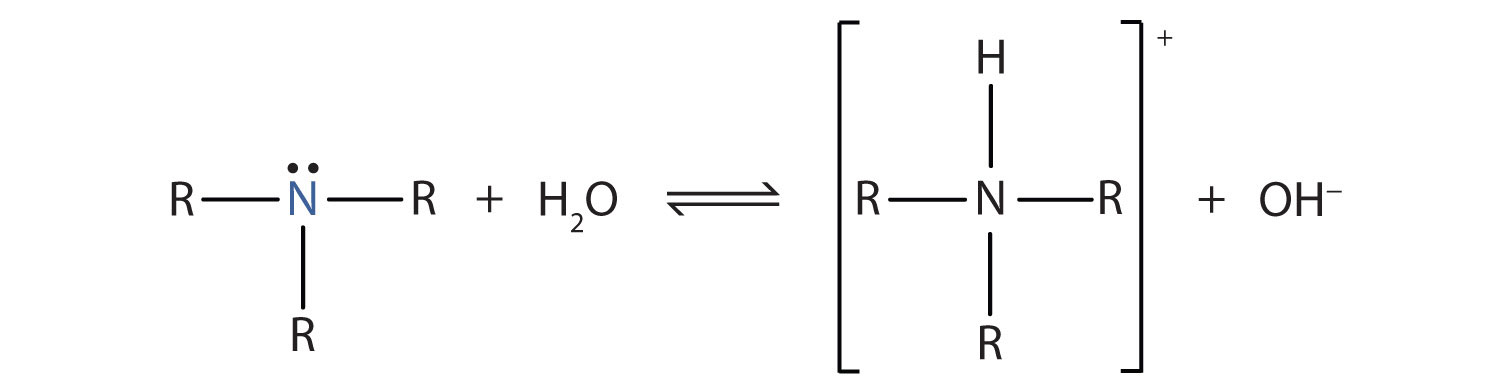
Draw the Lewis structure for the conjugate acid and base from the reaction of methylamine with a generic base (B:-). Include all lone pairs of electrons and any nonzero formal charges.

OneClass: Write a chemical equation for the hydrolysis reaction that explains why an aqueous solution...
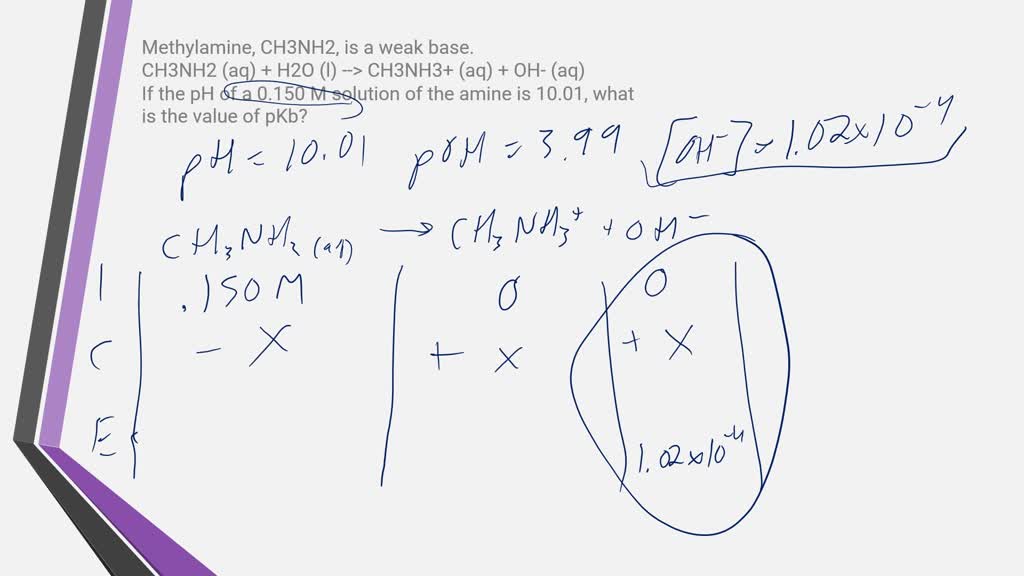
SOLVED: Methylamine, CH3NH2, is a weak base. CH3NH2 (aq) + H2O (l) –> CH3NH3+ (aq) + OH- (aq) If the pH of a 0.150 M solution of the amine is 10.01, what