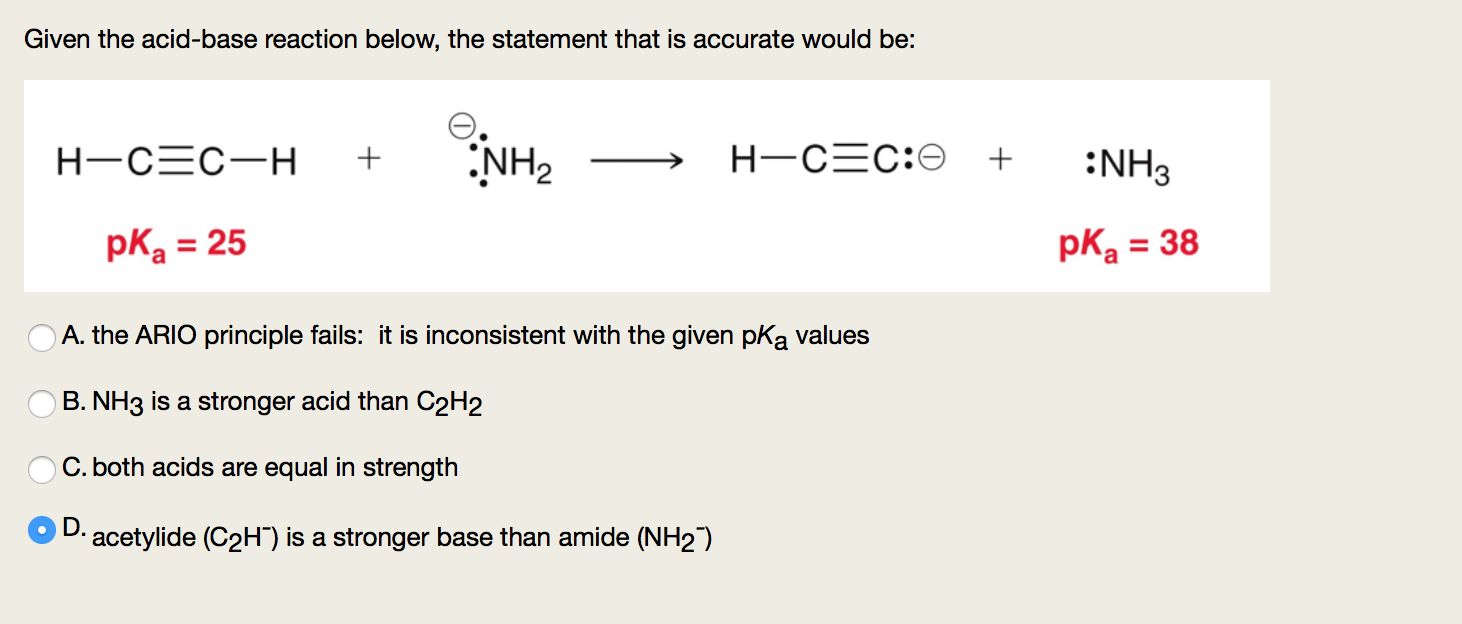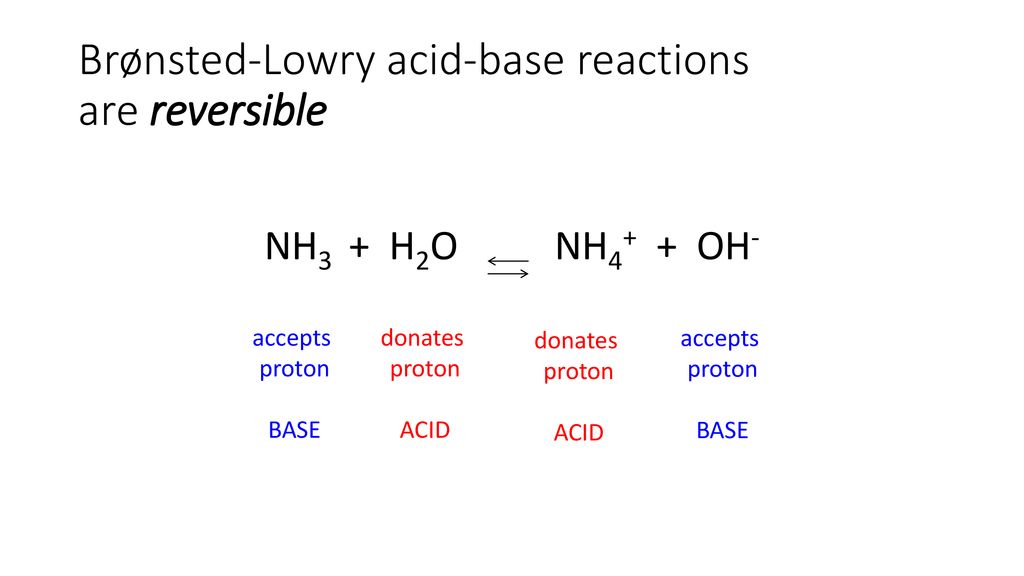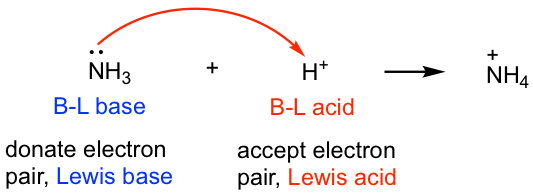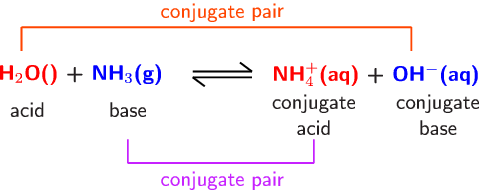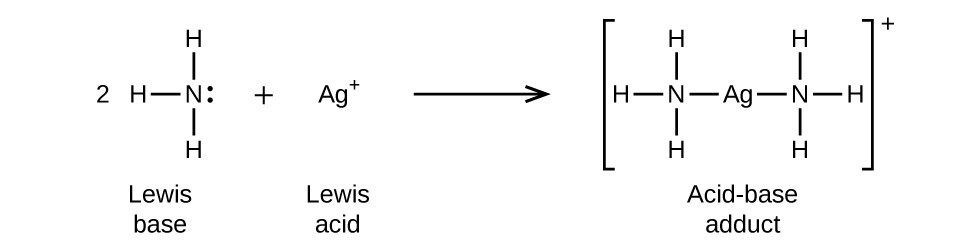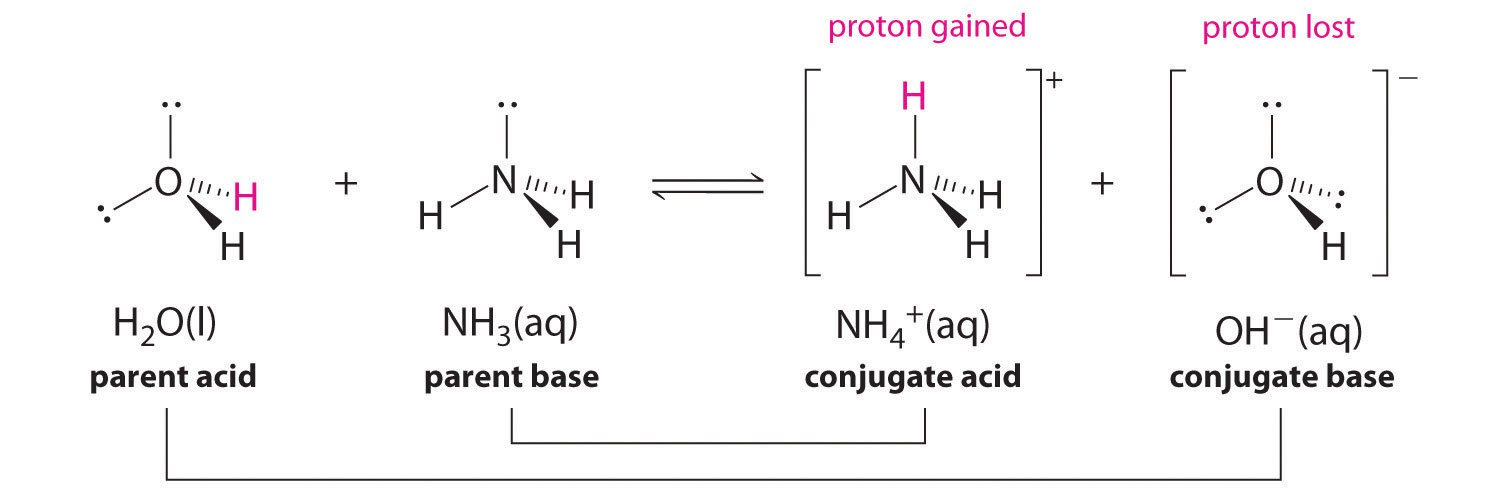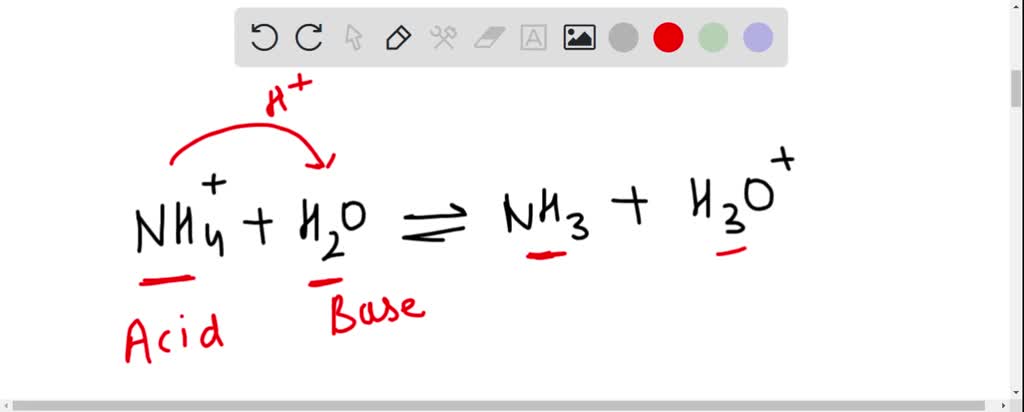
SOLVED: In the following reaction: NH4+ + H2O = NH3 + H3O+ A) H2O is a base and NH3 is its conjugate acid B) NH4+ is an acid and H20 is its
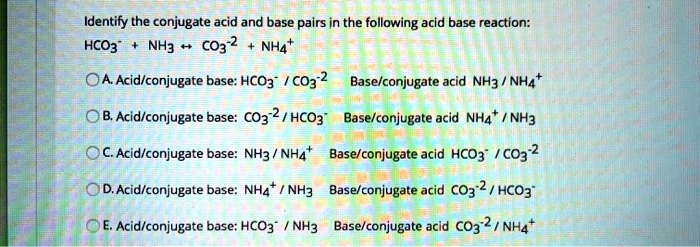
SOLVED: Identify the conjugate acid and base pairs in the following acid base reaction: HCO3 NH3 CO3-2 NH4t Acidlconjugate base: HCO3 CO3-2 Baselconjugate acid NH3 NH4 B Acidlconjugate base: CO3-2 / HCO3"