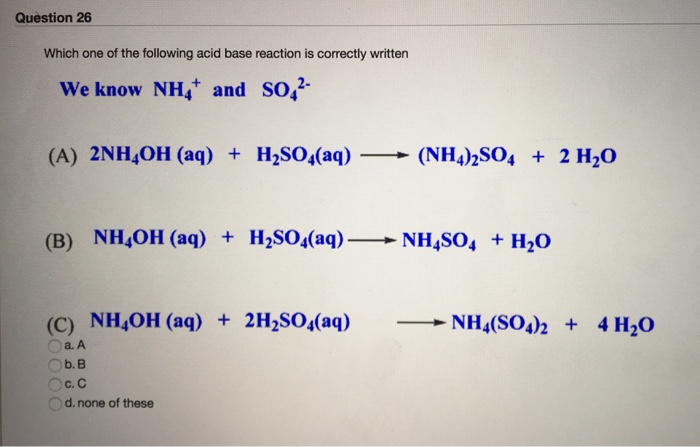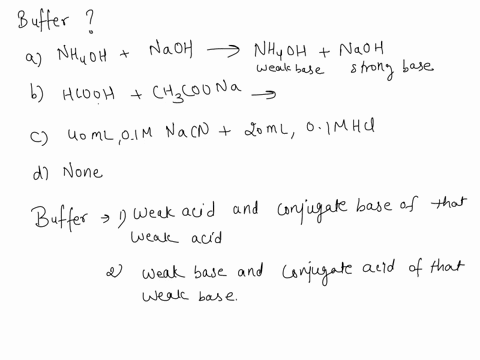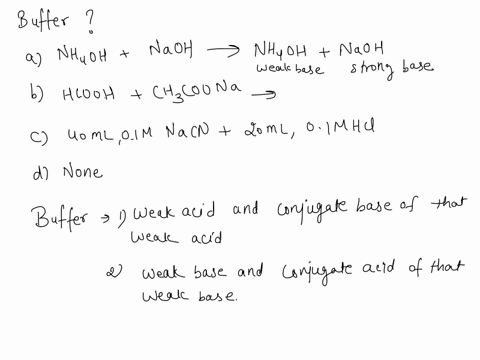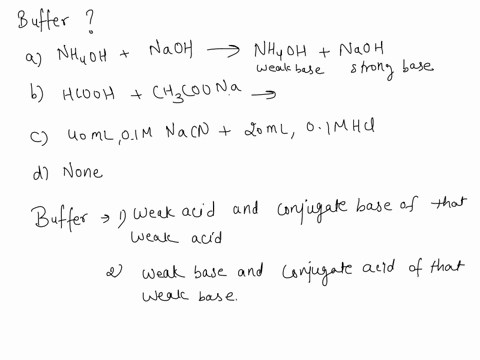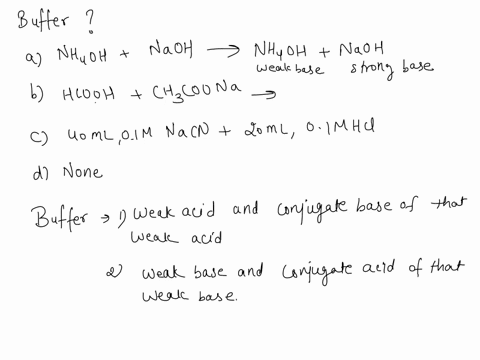
SOLVED: Which is a buffer solution? Select one: a. NH4OH + CH3COONH4 b. K2SO4 + H2SO4 c. NaOH + CH3COONa d. NaOH + Na2SO4

Question Video: The Net Ionic Equation for the Neutralization Reaction between Ammonium Hydroxide and Hydrochloric Acid | Nagwa

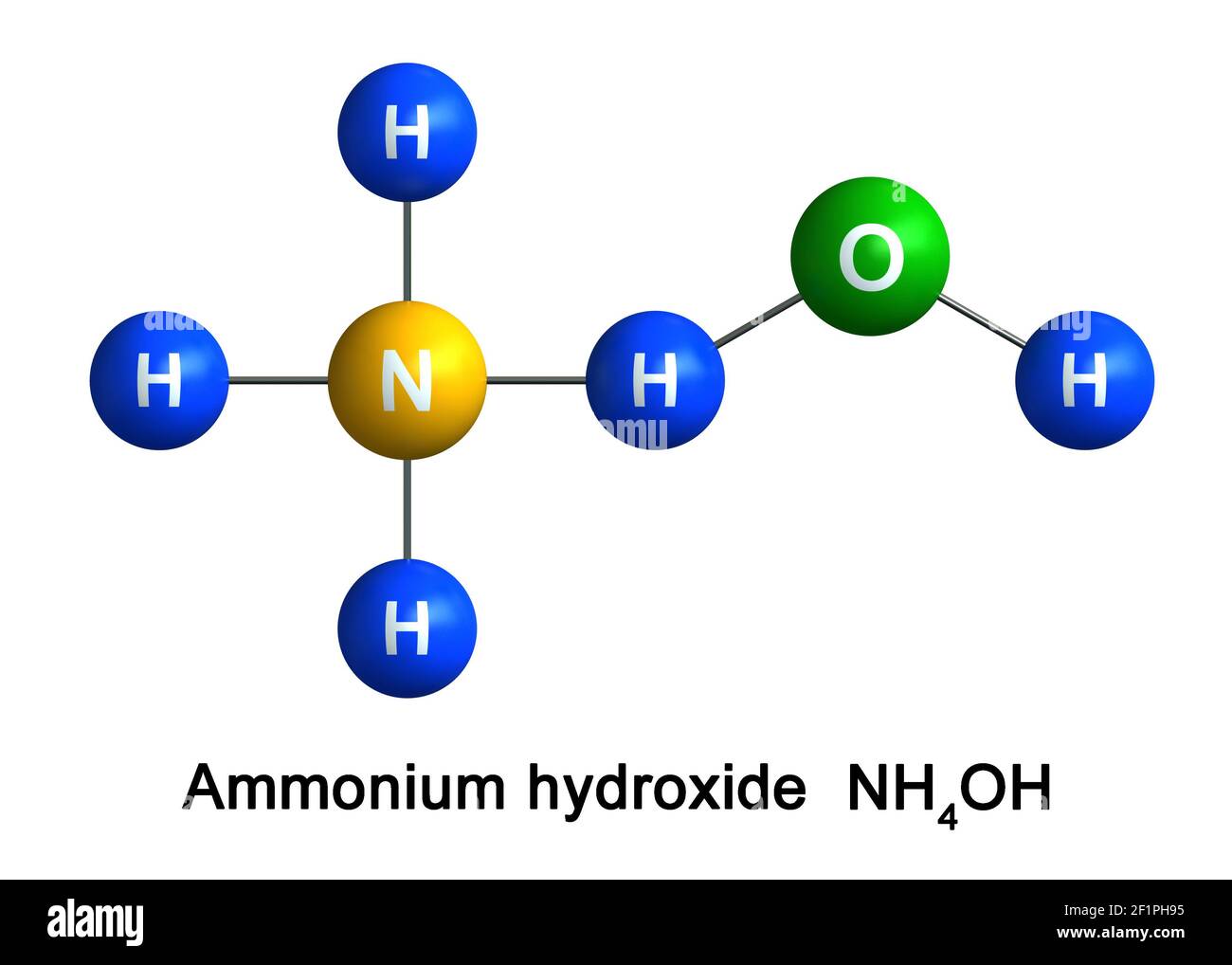
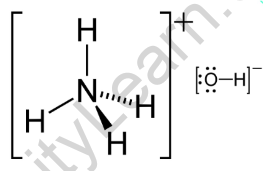
The pKb value of ammonium hydroxide is 4.75 An aqueous solution of ammonium hydroxide is titrated with HCl.The pH of the solution at the point where half of the ammonium hydroxide has