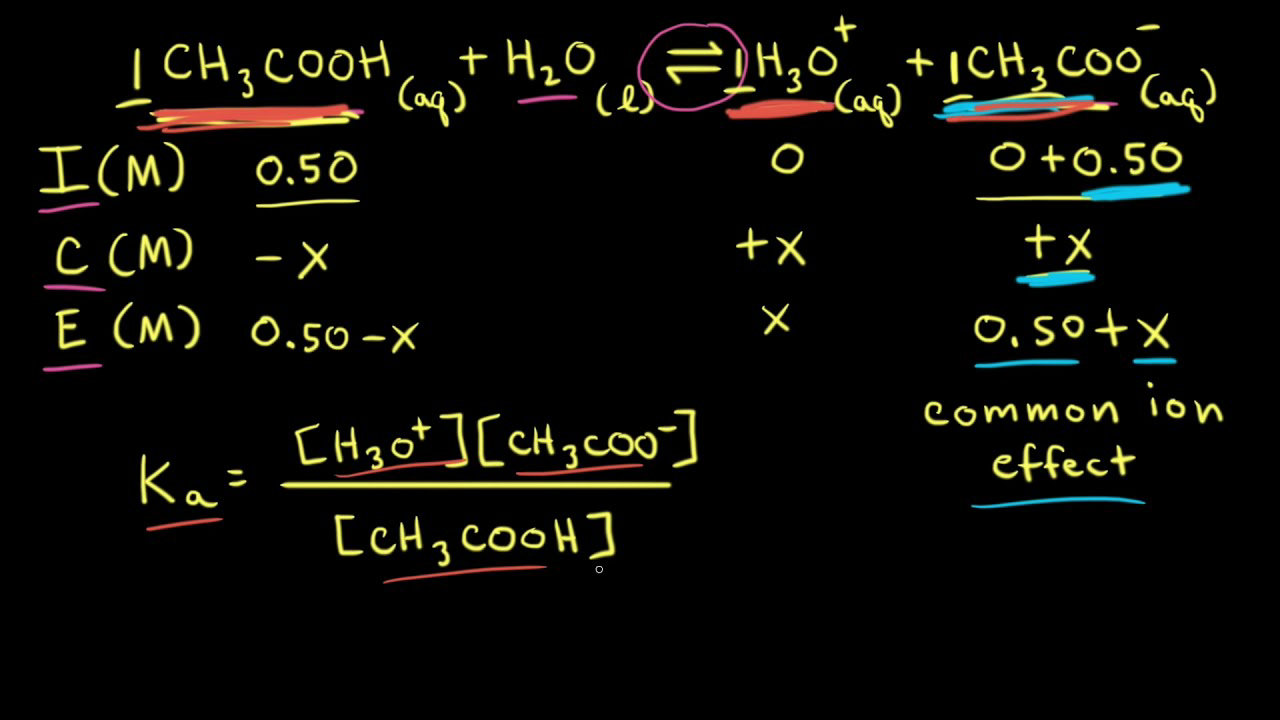
Worked example: Calculating the pH after a weak acid–strong base reaction (excess acid) (video) | Khan Academy

ph - Why is the gradient of the curve of a strong base titrated with strong acid small up until equivalence? - Chemistry Stack Exchange
3mL of a strong acid solution of pH=3 is mixed with 2mL of strong base solution of pH=11.What is the pH of new solution? Log(2)=0.301, log(5)=0.699.

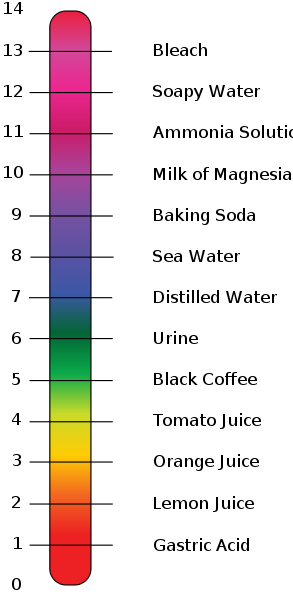

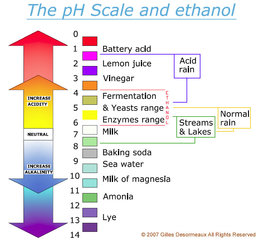
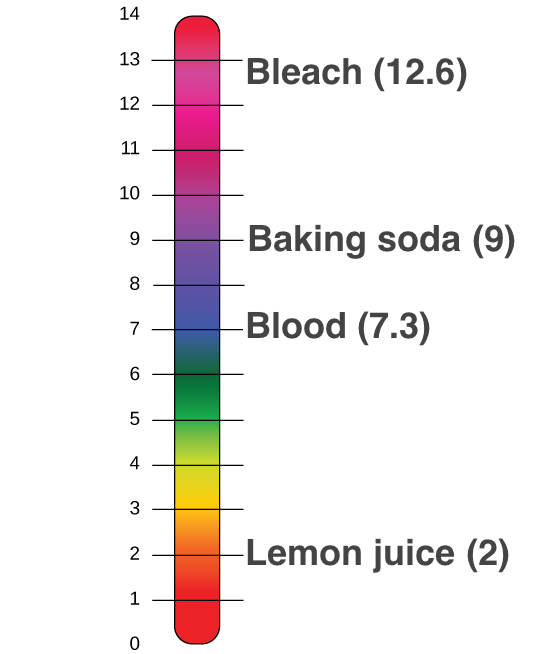
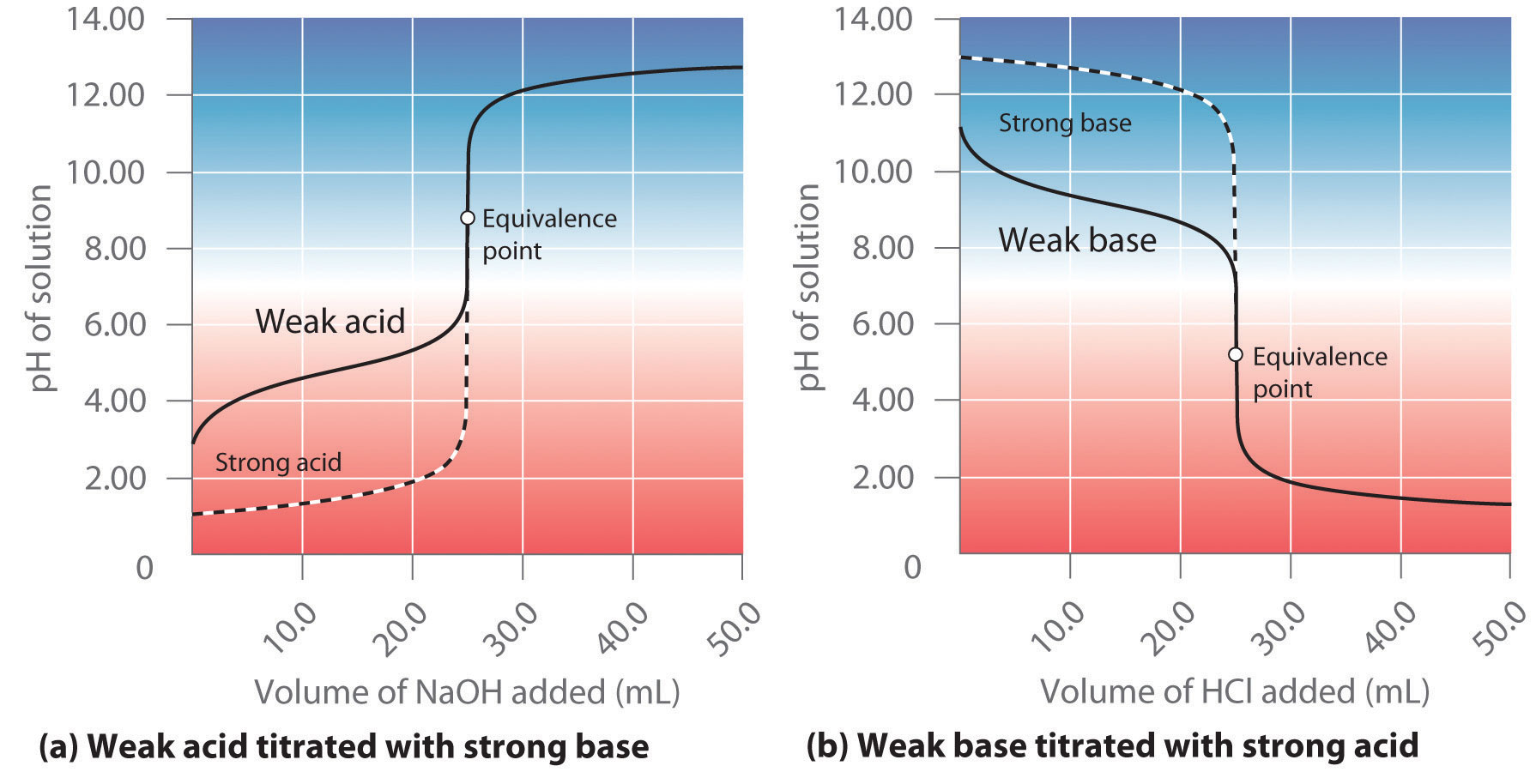
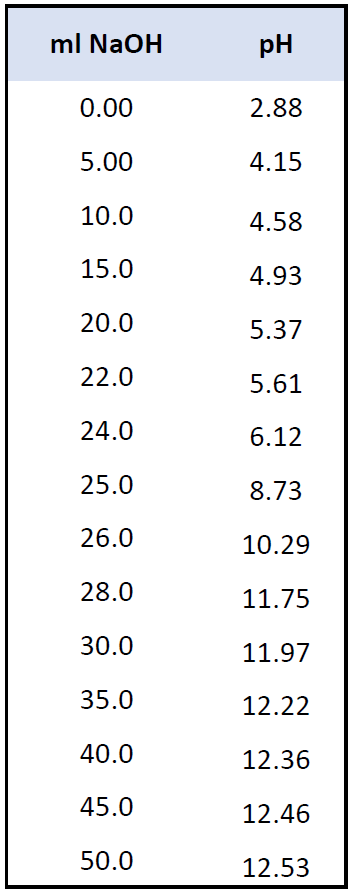





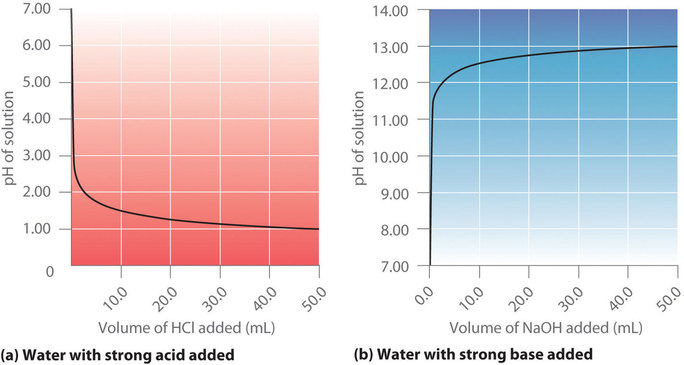



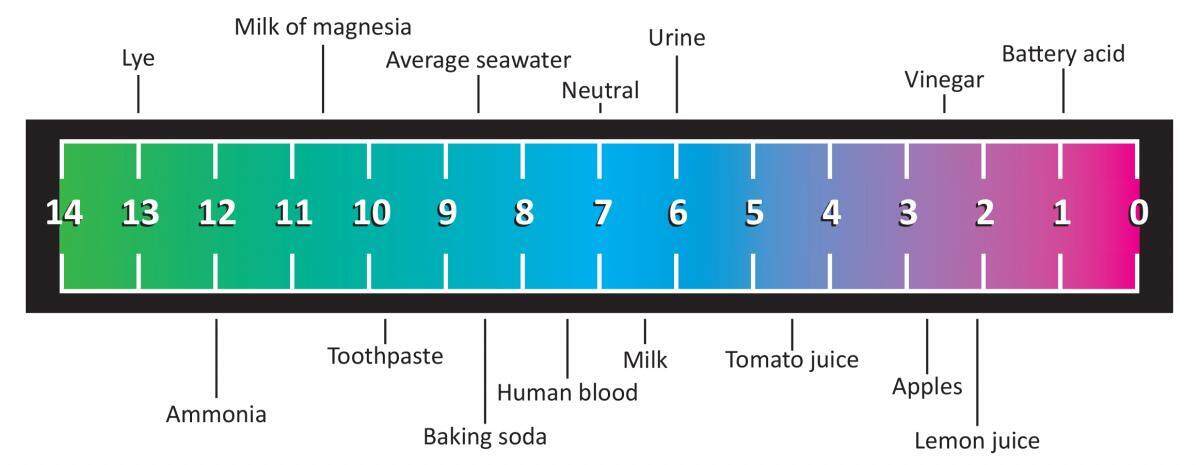
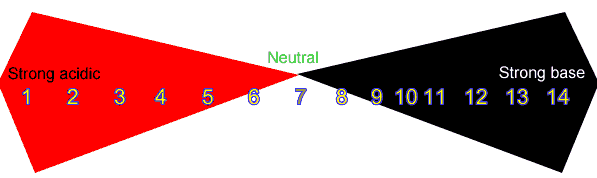


:max_bytes(150000):strip_icc()/168266757-56a131883df78cf772684a99.jpg)


