
Hion Calculator,12-Digit Large Display Office Desk Calcultors with Erasable Writing Table,Rechargeable Hand held Multi-Function Mute Pocket Desktop Calculator for Basic Financial Home School,White
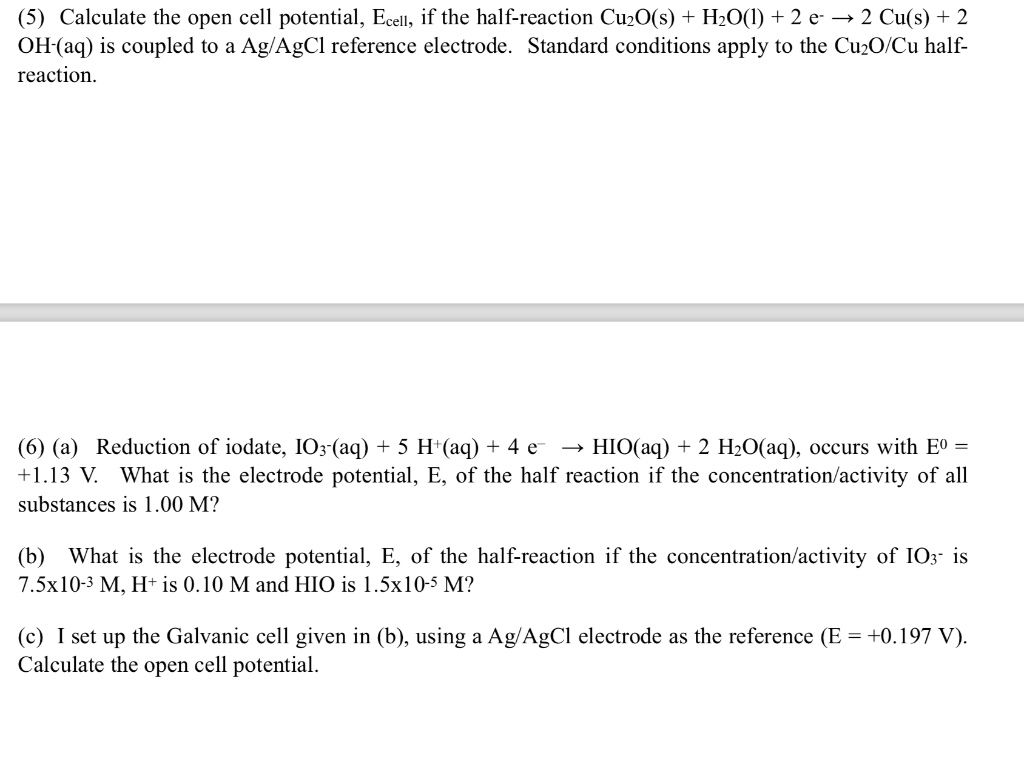
SOLVED: (5) Calculate the open cell potential, Ecell, if the half-reaction CuzO(s) + HzO(l) + 2 e 2 Cu(s) + 2 OH-(aq) is coupled to a Ag/AgCl reference electrode. Standard conditions apply
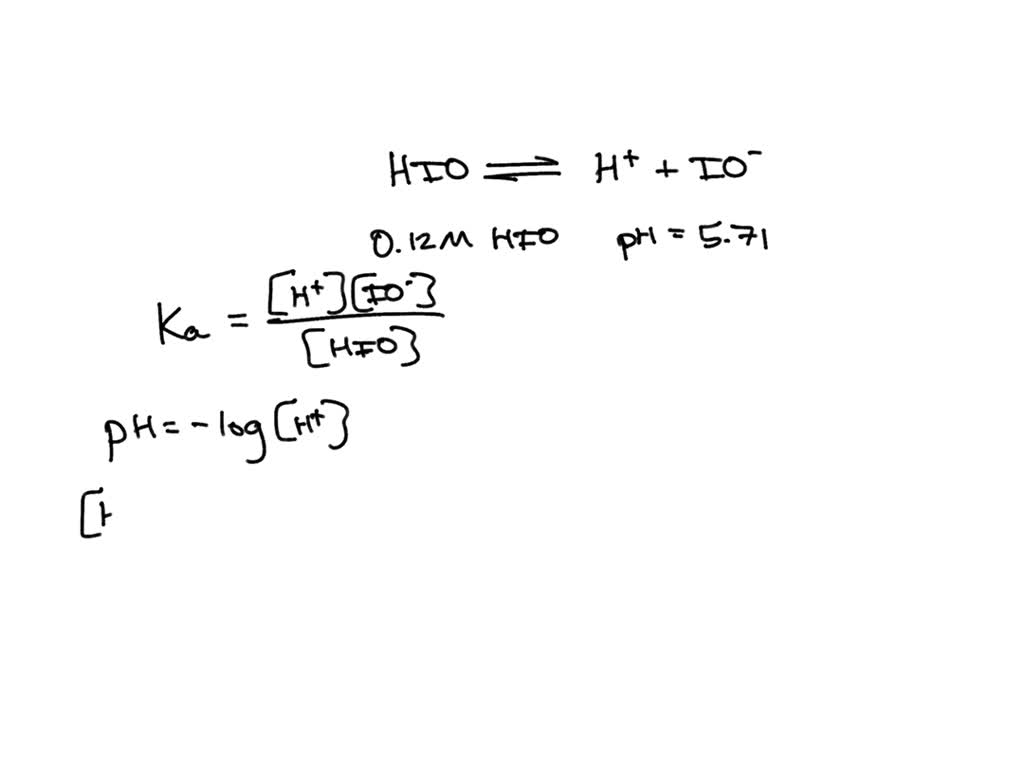
SOLVED: Hypoiodous acid (HIO) is a weak acid that dissociates in water as follows: HIO(aq) + H2O(l) H3O+(aq) + IO−(aq). A 0.12 M solution of hypoiodous acid has a pH of 5.71.
![SOLVED: Salts of hypoiodite ion behave as a weak base, undergoing hydrolysis in water according to the equation: IO- HzO = HIO + OH- Kb for hypoiodite ion 4.37x10-4 Calculate the [H+] SOLVED: Salts of hypoiodite ion behave as a weak base, undergoing hydrolysis in water according to the equation: IO- HzO = HIO + OH- Kb for hypoiodite ion 4.37x10-4 Calculate the [H+]](https://cdn.numerade.com/ask_images/9d80eb482c1a4947a4ffb060669f0832.jpg)












![Pangya Hole In One Calculator V10 [REPACK] - Machilipatnam Pangya Hole In One Calculator V10 [REPACK] - Machilipatnam](https://i1.wp.com/2.bp.blogspot.com/-4k7CoYQizQI/T78nWAhnc2I/AAAAAAAAACQ/kb2xm0AU9j0/s1600/ie8-beta2.png?w=696)
