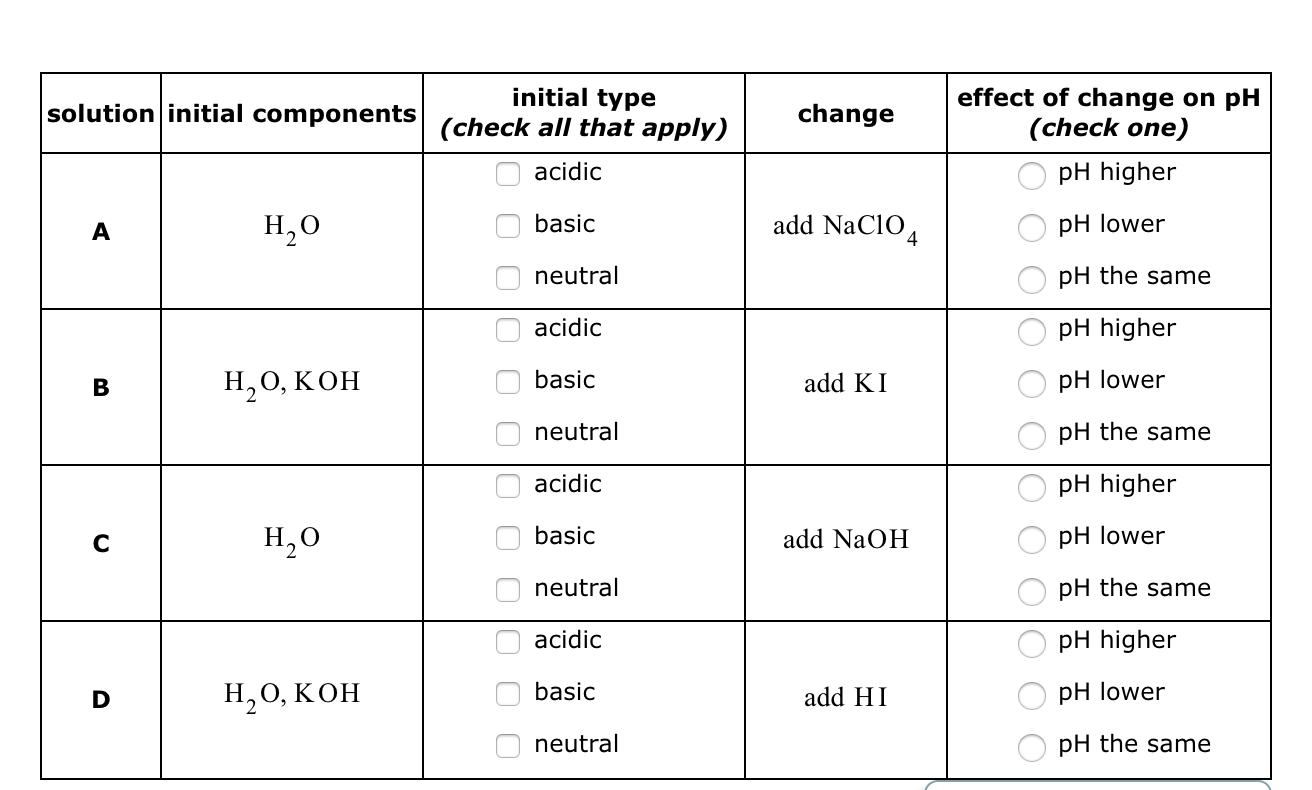
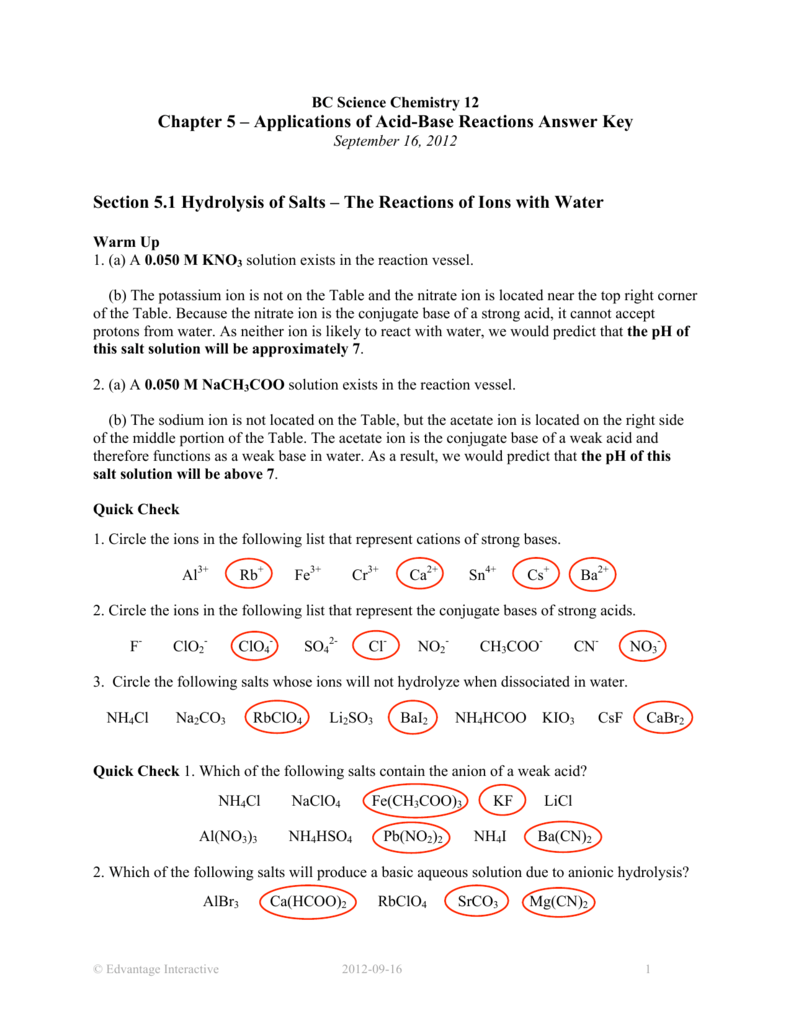
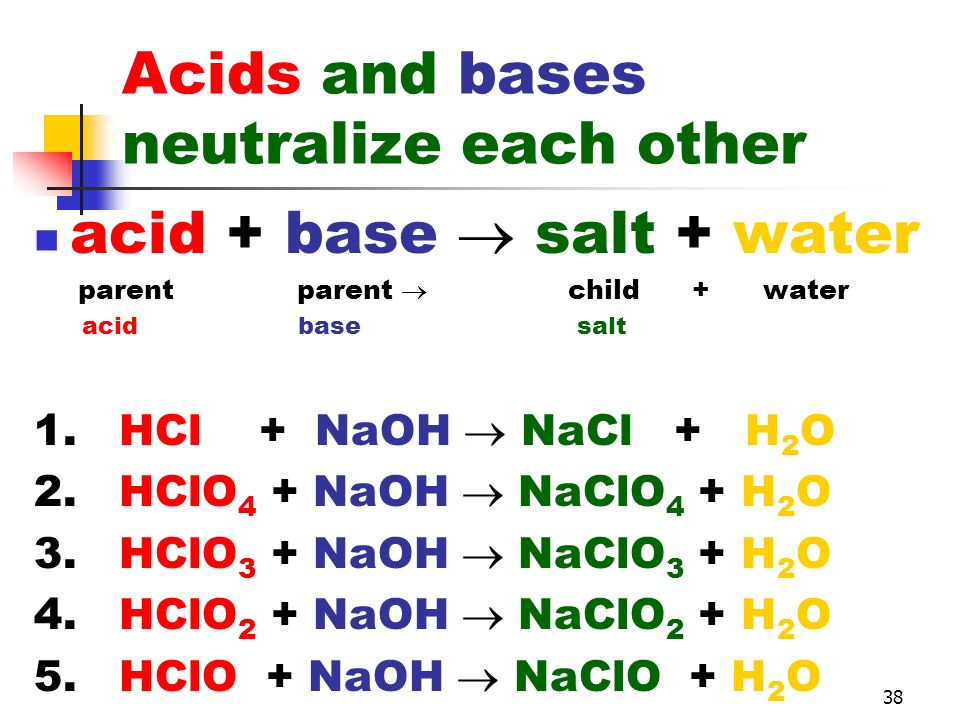
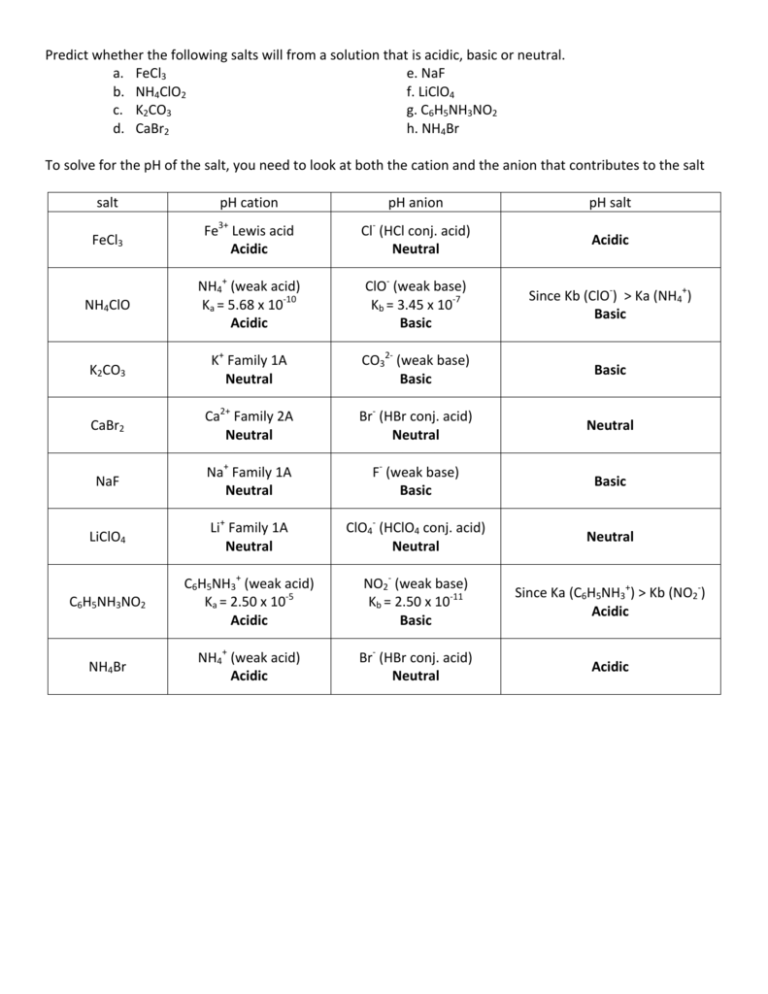
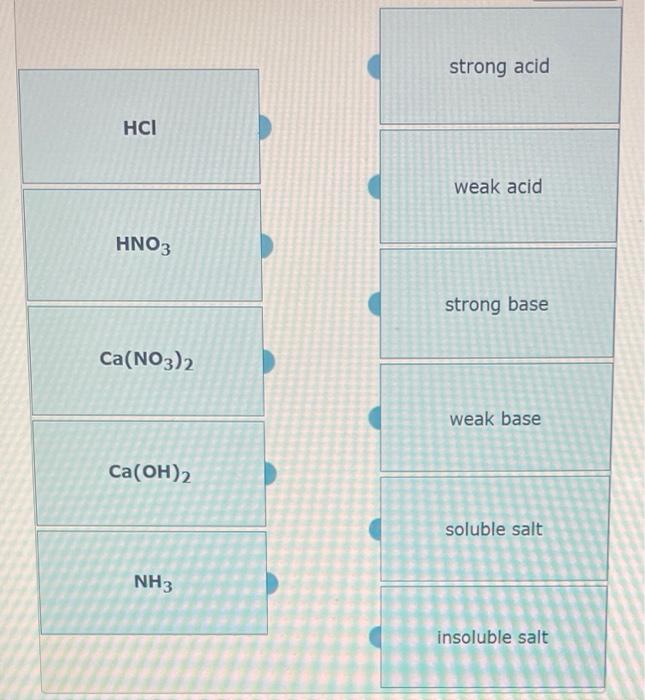
Predict if the solutions of the following salts are neutral, acidic or basic. NaCl, KBr, NaCN, NH4NO3,NaNO2 and KF
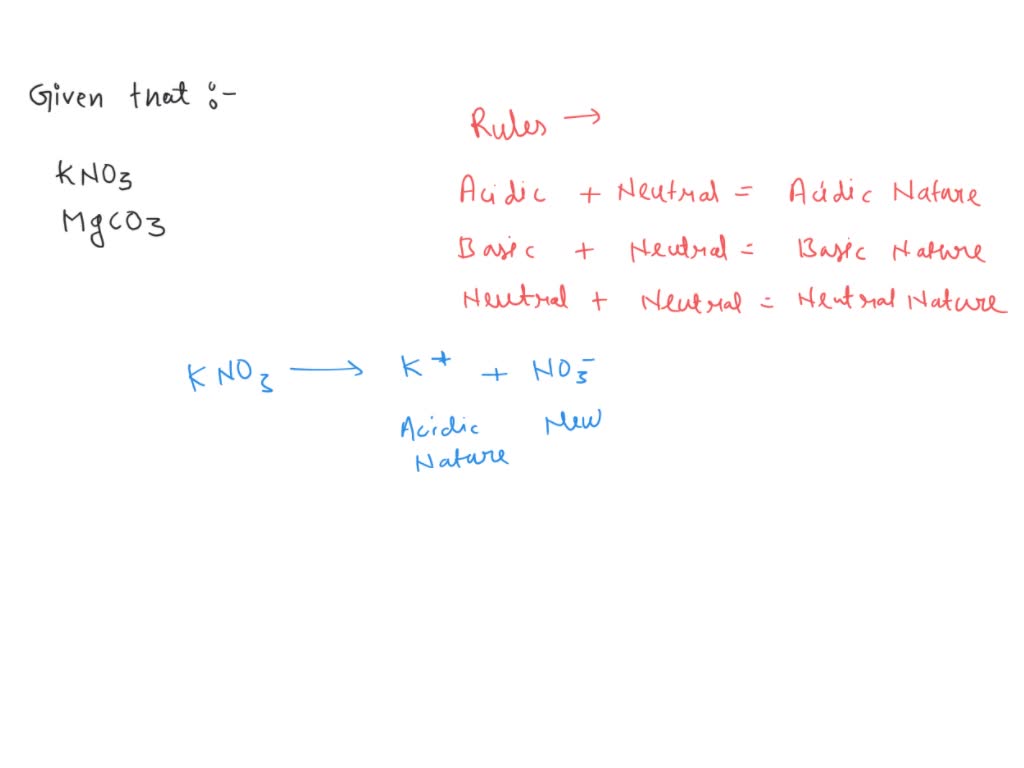
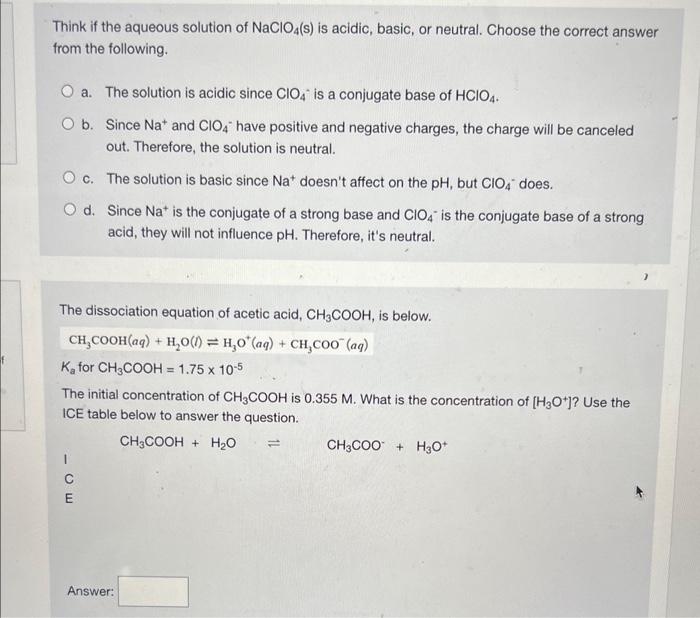
SOLVED: 'Classify each of the following as an acidic, basic, Or neutral salt, and then answer the following question Which @ne of the following is an acidic salt? KI b NaClO4 NHAI
Chem 321 Quiz 2 Name Answer Key 1. Identify what type of aqueous solution ( acidic, basic or neutral) each of the following s
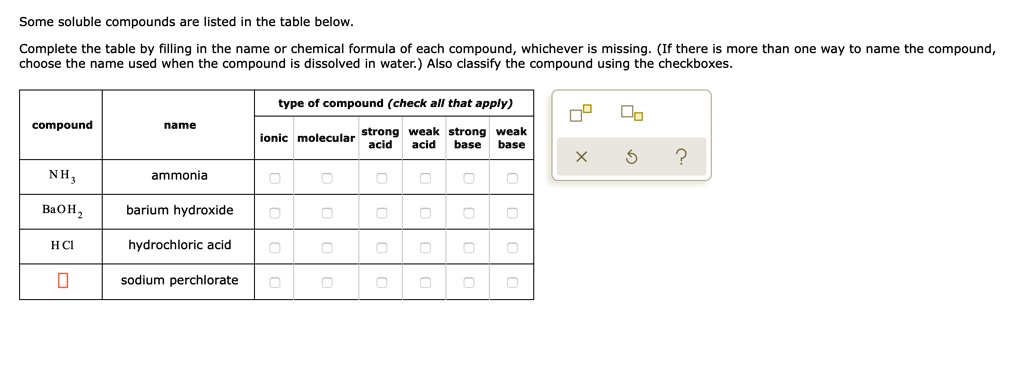
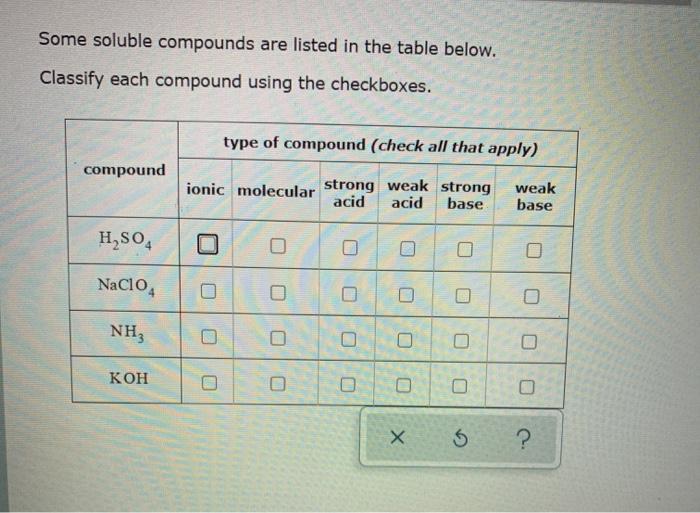
SOLVED: Some soluble compounds are listed in the table below Complete the table by filling in the name or chemical formula of each compound whichever is missing (If there is more than

Acids, Bases, and Salts You should be able to Understand the acid-base theories of Arrhenius, Brønsted-Lowry, and Lewis. Identify strong acids and. - ppt download