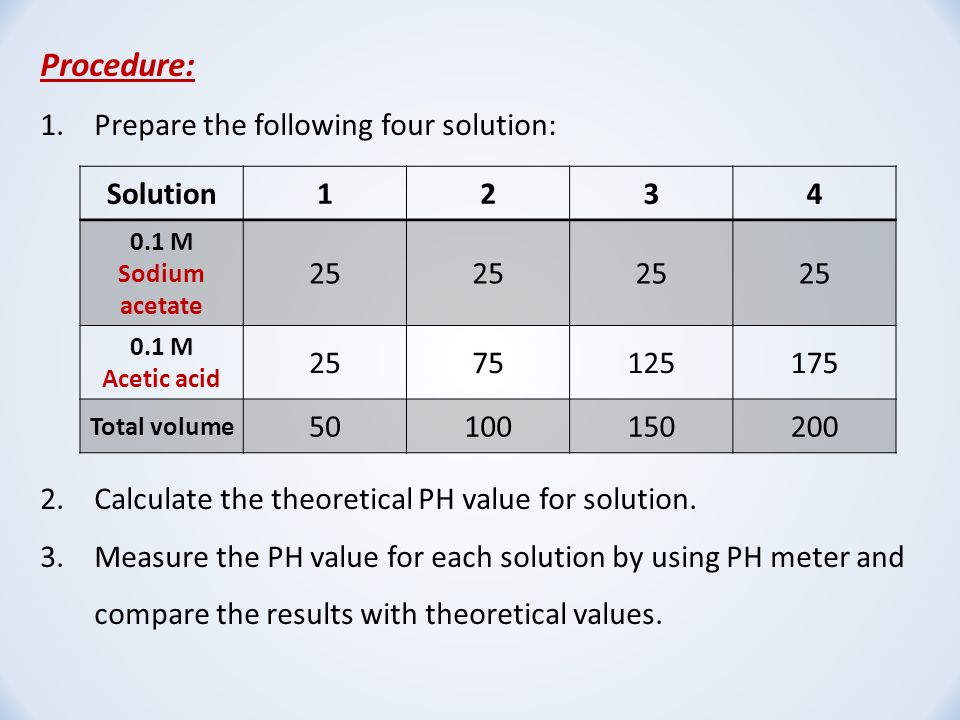
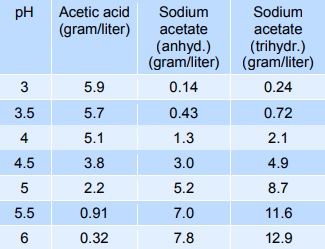
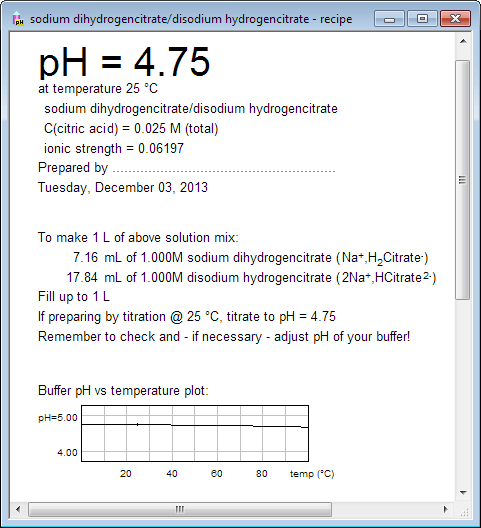
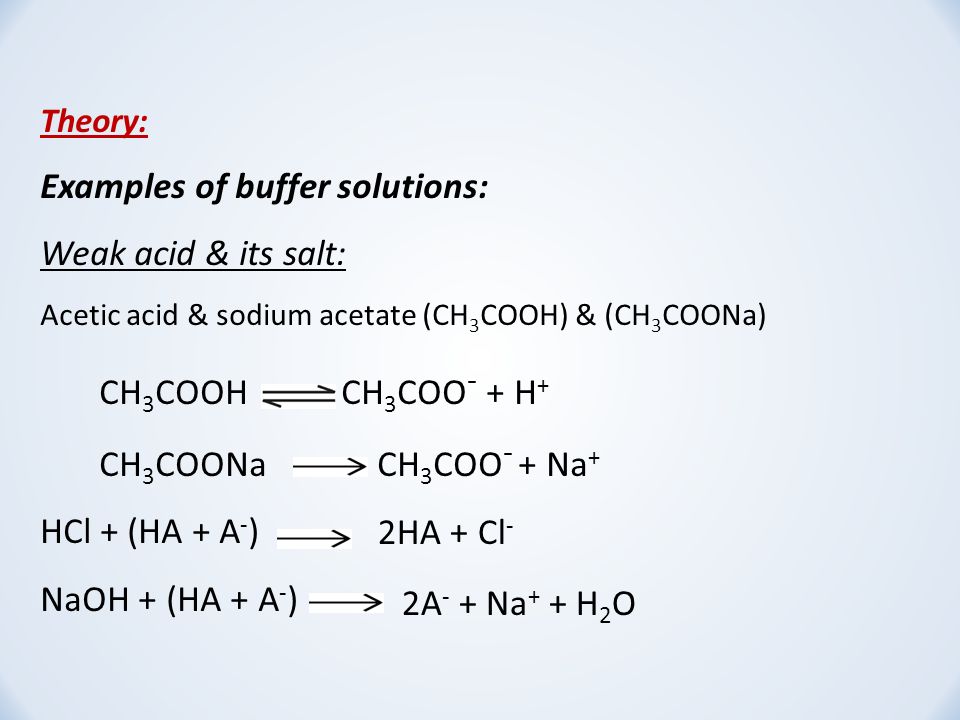
SOLVED: Question: Buffer question A) Calculate the pH of 1.00 L of a buffer containing 0.200 mol of acetic acid (HCzH3O2) and 0.250 mol of sodium acetate (NaCzH3Oz). Ka of acetic acid

ACETATE BUFFER SOLUTION PH 4.65, SODIUM ACETATE / ACETIC ACID, Honeywell Fluka™ 1L PLASTIC BOTTLE ACETATE BUFFER SOLUTION PH 4.65, SODIUM ACETATE / ACETIC ACID, Honeywell Fluka™ | Fisher Scientific

E-Lifes: Acetate buffer preparation and calculation. (Weak acid + salt of weak acid , Weak acid + salt of weak acid, Weak acid + strong acid)

Acid-Base Buffers Equation & Examples | How to Calculate pH of a Buffer - Video & Lesson Transcript | Study.com

Calculate the pH of `0.05M` sodium acetate solution, if the `pK_(a)` of acetic acid is `4.74`. - YouTube
![Calculate pH of a buffer prepared by adding 10 mL of 0.10 M acetic acid to 20 mL of 0.1 M sodium acetate. [pKa (CH3COOH) = 4.74 ] Calculate pH of a buffer prepared by adding 10 mL of 0.10 M acetic acid to 20 mL of 0.1 M sodium acetate. [pKa (CH3COOH) = 4.74 ]](https://i.ytimg.com/vi/t9B5VgPOTG4/maxresdefault.jpg)
Calculate pH of a buffer prepared by adding 10 mL of 0.10 M acetic acid to 20 mL of 0.1 M sodium acetate. [pKa (CH3COOH) = 4.74 ]

Acetate buffer preparation and calculation. (Weak acid + salt of weak acid , Weak acid + salt of weak acid, Weak acid + strong acid) | E-Lifes
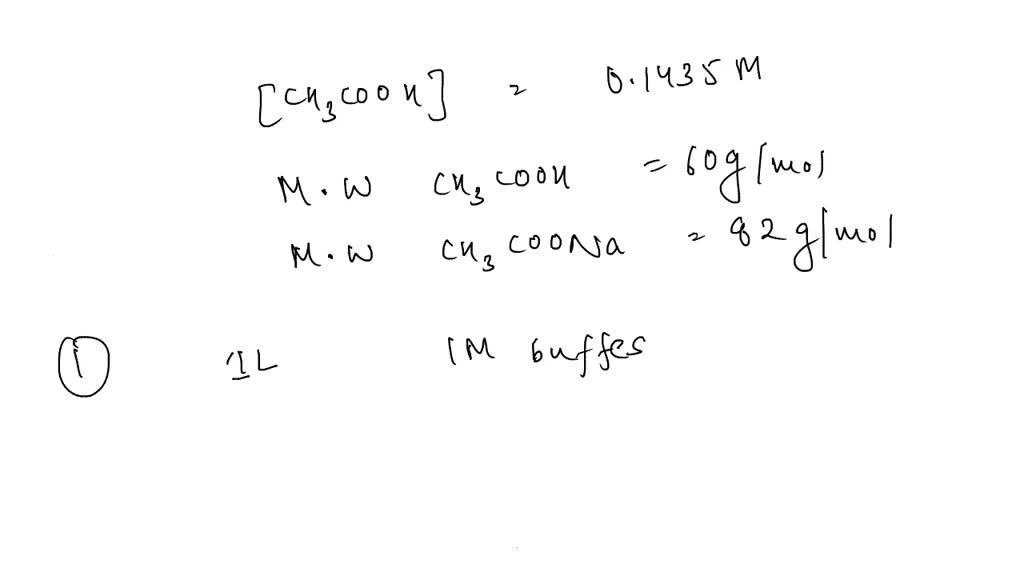

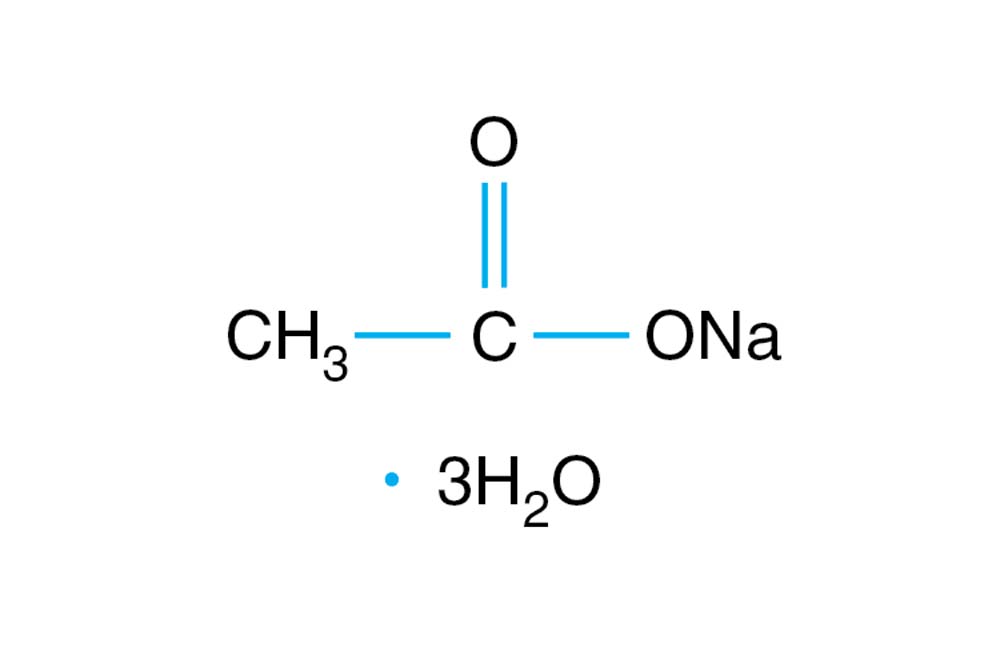
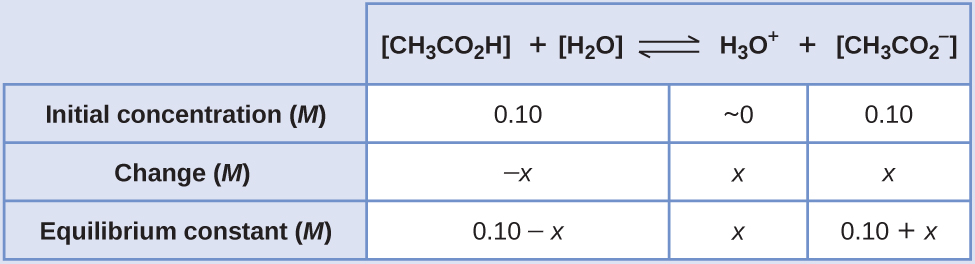

![Sodium Acetate Trihydrate [CH3COONa.3H2O] Molecular Weight Calculation - Laboratory Notes Sodium Acetate Trihydrate [CH3COONa.3H2O] Molecular Weight Calculation - Laboratory Notes](https://www.laboratorynotes.com/wp-content/uploads/2022/12/sodium-acetate-trihydrate-molecular-weight-calculation-300x228.jpg)




![Sodium Acetate [CH3COONa] Molecular Weight Calculation - Laboratory Notes Sodium Acetate [CH3COONa] Molecular Weight Calculation - Laboratory Notes](https://www.laboratorynotes.com/wp-content/uploads/2021/11/sodium-acetate-molecular-weight-calculation.jpg)